Vets, researchers dispute claims of lanosterol eye drops curing cataracts in dogs
The National Parks Board warns pet owners against self-treatment, cautioning that this could delay proper care and worsen their pets' conditions.
Eye drops being applied on a dog with cataracts. (File photo: iStock)

This audio is generated by an AI tool.
SINGAPORE: Veterinarians and pet owners are raising concerns over claims that off-the-shelf eye drops could cure cataracts in dogs.
The eye drops, which contain the ingredient lanosterol, are readily available online and in veterinary clinics in Singapore and they are being marketed by various brands as an effective cataract treatment.
Cataracts occur when the lens of a dog's eye becomes cloudy, blocking light from reaching the retina, potentially leading to blindness.
Researchers and vets told CNA that using the eye drops could delay surgery, which they said is the only treatment option for cataracts, and there is no solid scientific evidence supporting the efficacy of lanosterol eye drops in reversing the condition.
The popularity of lanosterol, a chemical compound, as a potential cataract treatment stemmed from a 2015 study suggesting that it could dissolve cataracts.
However, Dr Sara Thomasy, a professor at the University of California, Davis, told CNA that the study used a "subjective grading scheme" and lacked robust scientific validation.
Dr Thomasy, who is in the university's department of ophthalmology and vision science, also pointed out that lanosterol has poor solubility in water, limiting its ability to penetrate the eye and reach the lens where cataracts develop.
Singapore-based veterinary ophthalmologist Gladys Boo said that further research also refuted the effectiveness of lanosterol. A 2019 study demonstrated that the compound "failed to restore lens clarity", she added.
Despite these findings, lanosterol-based eye drops remain widely available under brand names such as Lanomax, CatarClear and LumenPro, with prices ranging from S$87 to S$159 (US$65 to US$120) for a 10ml bottle. They are sold on e-commerce platforms such as Amazon, Lazada and Shopee.
"DID NOTHING TO CLEAR THE CATARACTS"
CNA spoke to four pet owners in Singapore who used lanosterol-based eye drops, all saying the products did not improve their dogs' conditions.
Ms Audrey Bong, who is in her 40s and works in a professional services firm, bought lanosterol eye drops from her vet in 2020.
"At that time, I did have my suspicions because the vet's assessment of my dog's eyes seemed too simple," Ms Bong said. "(There was) no special eye examination, no documentation."
In mid-2021, after she had used the eye drops for a while, she noticed a "visible white spot" in her dog's eye and decided to seek a second opinion. This other vet confirmed that the eye drops "did nothing to clear the cataracts".
By then, her dog's condition had worsened, requiring more extensive surgery.

Ms Fiona Foo, 55, shared a similar experience. She used lanosterol eye drops on her dog for six months in 2021 but did not see any improvements.
She bought eight bottles of eye drops costing more than S$1,000 in total at the time, hoping that they would improve her dog's cataracts.
"It didn't become worse in that six months, but I didn't see any improvement or clearer eyes," the founder of animal welfare group Hope Dog Rescue said.
She also said that even though the eye drops were ineffective, she has about five friends who are still using them on their pets.
"I think they are scared to stop, in case (their dogs) become blind quicker."
HOW IT AFFECTS DOGS
Dr Boo, who runs The Eye Specialist for Animals clinic, said that she has treated about 20 dogs and their owners had used lanosterol products on them.
“Out of the 20, I could salvage four of the patients’ eyes,” she recalled. “For the rest, we needed to remove the eyes.”
She explained that cataracts in dogs often develop into glaucoma, which can lead to vision loss if not treated effectively. It can also cause the eyes to swell, causing pain to the dog, ultimately necessitating eye removal.
Although lanosterol does not directly harm the eyes, Dr Boo warned that it delays proper treatment in the form of surgery.
“When a pet owner is lulled into a false sense of confidence in the efficacy of lanosterol, the condition of the pet's eye will continue to worsen until the eye is effectively lost,” she said.
Dr Sandhya Nair from Oasis Vet clinic estimated that 10 to 20 per cent of cataract cases she had encountered involved pet owners who had tried lanosterol or similar products.
“These drops don’t directly cause a problem, but pet owners are misguided that the drops are helping their pets when in reality, the pet is not getting the right treatment,” she said. “The only and best way to treat cataracts is by surgery.”
Dr Cathy Chan from The Animal Doctors clinic echoed these concerns, saying that she has seen one client who tried it with her dog and another two who tried it with their rabbits.
She said that it is difficult to convince pet owners not to use the products.
“With products that do not have much scientific evidence, I usually discourage owners in their use but they are still easily available, so the clients are able to purchase on their own.”
CNA has contacted several vets who sell lanosterol online, but did not receive a response.

MANUFACTURERS DEFEND PRODUCTS
Despite expert scepticism, firms producing lanosterol eye drops continue to claim efficacy.
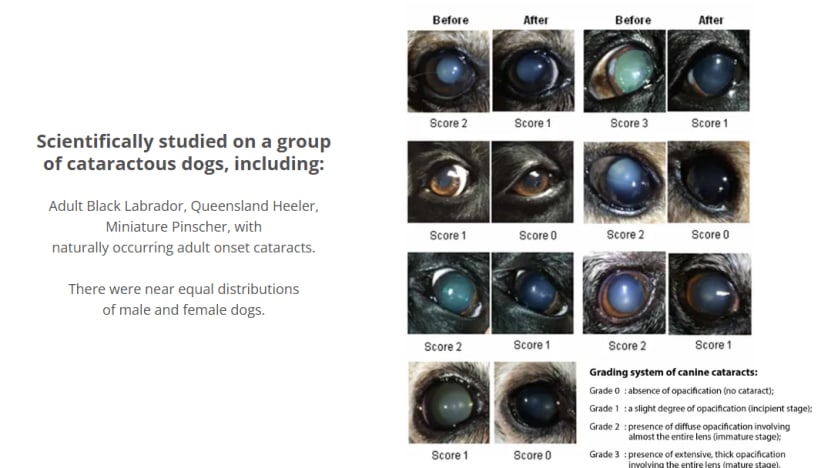
CatarClear states on its website that a survey of over 100 cataractous dogs showed a 98 per cent improvement rate.
However, when asked for details, its parent company Singapore Sciences clarified that the survey was based on "real-world veterinary experience" rather than a controlled laboratory study.
“The survey data on CatarClear supports positive outcomes,” Singapore Sciences stated.
Lanomax also defended its product, acknowledging that lanosterol "cannot penetrate the cornea" but asserting that its proprietary "lanosterol delivery system" overcomes this limitation.
The company also cited a 2018 case study where Lanomax cured cataracts in one human patient.
CNA contacted another eye-drops manufacturer, LumenPro, but has not received a response.
EXPERTS CALL FOR SCIENTIFIC RIGOR
Researchers remain unconvinced by the manufacturers' claims. Dr Eric Ledbetter, a professor of ophthalmology at Cornell University in the United States, pointed out that the CatarClear survey does not appear to have been subjected to peer review and published in a credible scientific journal.
“Almost no details about the study design or its results are provided on the website to support the claims … Obvious and potentially relevant financial interests and conflicts of interest are not clearly disclosed,” Dr Ledbetter told CNA.
“An appropriately designed, controlled and masked study in dogs with cataracts is necessary to establish the effectiveness of lanosterol to treat canine cataracts.”
On the study presented by Lanomax, Dr Boo from Singapore said that it was based just on one human patient.
"A proper study requires a reasonable sample size and a control group," she added. "These standard measures ensure that conclusions drawn from the study are not based on random chance."
She also highlighted that the study measured only the concentration of lanosterol in the patient's tears, but did not establish the concentration of lanosterol within the eye and lens.
"Therefore, there is no proof that lanosterol was of any therapeutic value," Dr Boo said.
Dr Ledbetter said that advocates for the use of lanosterol in dogs with cataracts should provide “more solid scientific evidence that their products are safe and effective”.
“Without this, the available ‘evidence’ appears to simply be marketing.”
The Animal & Veterinary Service, a cluster of Singapore's National Parks Board (NParks), is aware of different formulations of lanosterol eye drops being sold as veterinary health products here.
Dr Charlene Fernandez, group director of professional and scientific services at NParks, cautioned: “We strongly urge pet owners to take their pets to veterinarians for proper diagnosis and treatment for any health concerns.
“Pet owners should refrain from self-treatment for the safety and well-being of their pets because this may result in a delay in proper treatment, which may worsen the condition and health of their pets.”

















